From the number of views H2 Chemistry threads on this forum has
garnered, it's pretty clear that hundreds of lurkers (presumably
and hopefully the majority of whom are actual JC students) are
interested in H2 Chemistry posts.
Well, to all you lurkers out there (especially if you're a JC
student), here's a suggestion : Why don't *you* go ahead and post
your own H2 Chemistry queries here.
Just register an account with SgForums, and feel free (it's
literally free... of course, asking for free online help here... or
for that matter anywhere... isn't as effective as actually joining
my tuition, but it's still better than having no one reliable to
ask for help... or if you disagree with your own private tutor or
school teacher and wish to have a 2nd opinion that's reliable and
trustworthy) to ask anything relevant to H2 Chemistry here.
For instance, if you wish to discuss a TYS qn or a JC Prelim qn
(eg. if you don't understand or disagree with the answer given by
the TYS author, or the prelim paper's mark scheme, or your school
teacher's explanation. etc), you can just specify the question (eg.
"2014 RJC P3 Q2" or "TYS 2014 P3 Q2" etc) and ask your question. If
it's a non-TYS qn or non-Prelim qn that involves a diagram, you'll
have to use your handphone to take a photo, and upload the image to
your Facebook, Twitter, blog, etc, and link to the image in your
post.
Terms & Conditions : Only genuine and reasonable questions from
genuine and reasonable students asked in a genuinely and reasonably
respectful manner will be replied to.
![image]()
----------------------------------------
On Exam Smarts &
Question Ambiguity
Eg 1. What kind of reaction is
this?
CH3CH2OH + CH3COOH --> CH3CH2COOCH2CH3 +
H2O
Comments :
When faced with an ambiguous question like this, the
exam-smart student will give all 4 of the following answers
:
1) Esterification reaction
2) Condensation* reaction
3) Addition-Elimination** reaction
4) Nucleophilic Acyl Substitution**
reaction
(*Note : "dehydration reaction" not
acceptable; dehydration removes water from one molecule, while
condensation removes water or any small molecule from
two molecules to combine them together)
(**Note : "nucleophilic substitution" alone is not
acceptable because it implies nucleophilic aliphatic substitution
which occurs by an SN1 or SN2 mechanism. When a
substitution occurs on an acyl group, it's called a nucleophilic
acyl substitution and the mechanism by which this occurs is called
addition-elimination.)
Why give 4 answers? Because all 4 are indeed
correct and relevant, and each describes a different quality of the
same reaction (eg. is a fresh apple red, round, juicy or
edible? It's all four!). However, the question setter and mark
scheme might have been looking for specifically one out of the
four, which unfortunately due to the question ambiguity you're
left guessing which, so the only smart thing to do is
to provide all 4 details (students who only write
one out of the four possible answers, would in a sense have
only a 25% chance of securing the mark, depending on how
reasonable the mark scheme is).
If the question involves generating an amide (instead of
an ester), as is the case with the 2011 'A' level exam paper,
then the correct answer would be "nucleophilic acyl
substitution; addition-elimination;
condensation."
Another common ambiguity issue with such questions
"So-much of a hydrocarbon is exploded with so-much of oxygen,
and when the residual gases were cooled to r.t.p., there was
an expansion/contraction of so-much. What's the formula of the
hydrocarbon?"
If you interpret the question to be (the
expansion/contraction) referring in regard to reactant gases,
you end up with one formula answer. If you interpret the question
to be referring in regard to residual gases, you end up with
another formula answer.
Specific Example, Eg.3 :
"When 20cm3 of a gaseous hydrocarbon was sparked with
150cm3 of oxygen and the residual gases cooled to rtp, a
contraction of 60cm3 occurred. A further contraction of 80cm3 took
place when the residual gases were subjected to aq NaOH. All
volumes measured at 20 deg C. Determine the formula of the
hydrocarbon."
If you interpret the contraction referred to residual
gases, you obtain the answer (formula of hydrocarbon) as
C4H6.
If you interpret the contraction referred to reactant
gases, you obtain the answer (formula of hydrocarbon) as
C4H8.
The exam-smart student will give both alternative
workings & answers (but with
clear labels/qualifications/explanations to your alternative
answers! Otherwise the marker will think you're trying to cheat by
giving multiple answers and you get zero marks!). Some students
protest, "but as it is we already don't have enough time to
complete the paper!". Students do not have enough time to complete
the paper only because they spend too much time on questions which
they are stuck or don't know how to do. If you encounter such
questions, the exam-smart thing to do is skip! (and get back to it
later). But spending the little extra few seconds to write out an
alternative interpretation answer (to an ambiguous question), will
secure your marks for that question and is well worth the time to
do so. Be exam-smart!
Conclusion :
When faced with an ambiguous question, the exam-smart
student will always give all possible alternative correct answers,
as long as you clearly qualify, label and explain them. (If
you don't, you may lose marks because the marker may think you're
trying to cheat by giving multiple answers to a single
question.)
Write a brief-but-polite note : "Dear Cambridge Marker
Sir/Mdm, with all due respect, the question is tragically ambiguous
as it is does not make clear whether... ... Hence, in the
interest of giving adequate coverage to the possible alternative
interpretations/assumptions, I will be providing both possible
working calculations or answers below. On the left below, I
assume/interpret the question as such-and-such. On the right
below, I assume/interpret the question as such-and-such. Thank
you for your kind understanding."
Of course, for questions such as example 1 above,
there's no need to write any explanation at all, simply write the
answer as, "This reaction is an esterification; condensation;
addition-elimination; type of reaction."
As long as all your alternative answer points are
all correct, and you qualify/explain them where necessary, you will
surely secure your full marks. This is being
exam-smart.
---------------------------------------
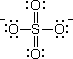
Originally posted by H4x0ru5:
Hi,
I would like to ask how charges for the polyatomic ion
such as SO4 2- is derived. Based on what I have found on the
internet, there are 2 explanations :
1. Formal Charges
To draw a Lewis structure for sulfate ion, there are 6 + 6(4) +
2 = 32 electrons for bonding
After doing the bonding and stuff, the two oxygen atoms that has
a single bond with sulfur has both 6-6-(2/2) = -1 charge each, thus
giving SO4 a negative charge of 2.
![]()
But there's something I don't understand. How do you derive a
charge of 2- in the first place without even adding the 6 +
6(4) + 2 = 32
? That's not valid. Cause if I simply give you SO4 how do you
even know you have to add another two more electrons to the
structure and derive the formal charges of each atom ?
That brings me to the next point,
2. Bonding
30 - 8 (4 bondings between each oxygen and sulfur atoms) =
22
Putting the 22 electrons in place, I find out that the last
oxygen atom lacks 2 more electrons to fulfil a stable
octet.
Thus I derive that SO4 has a negative charge of 2-
And I get the following diagram
![]()
My question is, which is right and which is wrong ?
This topic is something best dealt with in person during
tuition, and is difficult to explain over an online forum. I'll
give brief comments, do the best you can to understand them, and
anything further (eg. if you still do not fully comprehend
this topic) ask your school teacher or tuition teacher if you
have one.
I'll comment directly on your self-drawn structure, which should
go some way to auto-answer your previous queries.
First of all, these are more correctly called Kekule structures,
rather than Lewis structures (unsurprisingly, different chemists,
schools, examiners, use different terms).
Secondly (and you'll find some school teachers disagreeing on
this point; (unlke fussy school teachers) Cambridge actually
doesn't care about petty little details like this, and will accept
either presentation or method), you should always show all formal
charges within the square brackets, as well as
simultaneously show the ionic charge outside the square
brackets. Afterall, the square brackets symbolize summation (for
physical and inorganic chem purposes; we do not use square brackets
for organic chem purposes).
Accordingly, your drawing of the sulfate(VI) ion is erroneous in
that you omitted the formal charges on your atoms : a dipositive
formal charge on the S atom, and a uninegative formal charge on
each of the singly bonded O atoms.
Also you neglected to show dative bonds. Everytime you see
positive and negative formal charges on adjacent atoms, you should
suspect the presence of dative bonds.
(Interestingly, dative bond presentation is different for
all three : physical chemistry, inorganic chemistry and organic
chemistry. Again, different JC teachers will disagree with each
other on the best presentation for these, and again Cambridge
doesn't care so much, and will accept a variety of presentations.
Nonetheless, I'll briefly indicate the best presentations
:
physical
chem : show formal charges after dative bond
formation. And there should not be any lone pair at the base of the
dative bond arrow.
inorganic
chem (complex ions) : show formal charges
before dative bond formation. And there should be a lone pair at
the base of the dative bond arrow, on the ligand.
organic
chem : show a curved arrow (for which the lone
pair must be shown at the 'base' of the curved arrow) to
illustrate electron flow, and in the next stage of the mechanism, a
straight line (*no* dative bond arrowhead should be
shown) is used to indicate the bond formed.
The difference between physical and inorganic chem
presentations, arise from the fact that the electronegativity
difference between non-metals is small, but between metals and
non-metals is large. As such, formal charges after dative bond
formation is more accurate for species in which all atoms
are non-metals, but formal charges before dative bond
formation is more accurate for complex ions in which ligands are
significantly more electronegative than the metal ions they donate
dative bonds to)
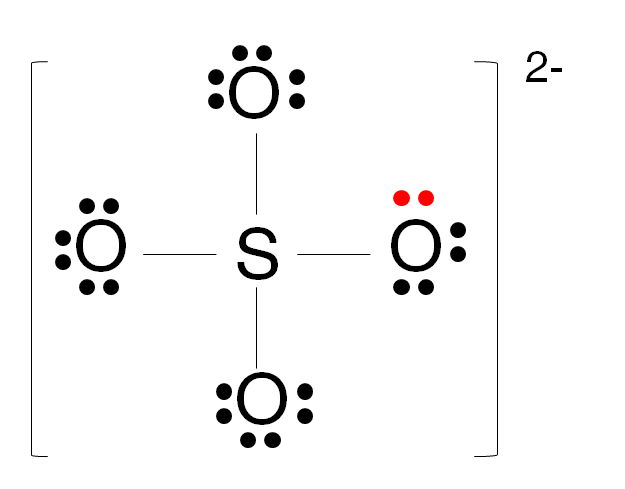
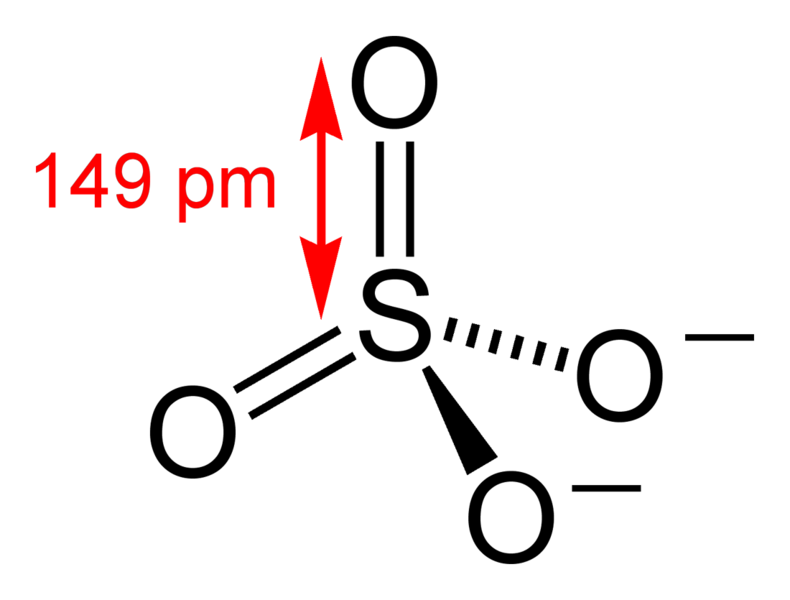
What you've drawn (ie. all singly bonded O atoms) is favoured by
US chemists, while UK chemists favour the expanded octet
version (ie. with some doubly bonded O atoms). If you could
shrink yourself to the size of an atom and observe the sulfate(VI)
ion, which structure would you see? The US version or the UK
version?
Tis a trick question. Both versions are equally correct, because
they are really resonance
contributors of each other. The actual structure
(that you would see, if you could shrink yourself to the size of an
atom) is known as the resonance hybrid, and
has partial double bond character for all 4 bonds with all 4 O
atoms.
Cambridge will accept either the UK or the US presentation, but
of course, the UK presentation is recommended for Cambridge 'A'
level students. Note however, for period 2 elements (since they do
not have vacant, energetically accessible 3d orbitals to use to
expand their octet), both UK and US structures are the same.
For the UK presentation, this is how you draw the Kekule
structure of the sulfate(VI) ion :
Ionic charge is the sum of formal charges.
Since the ionic charge is dinegative, we assume two of the O
atoms have a uninegative formal charge.
This means these two O atoms must have 1 bond pair and 3 lone
pairs.
The other two O atoms must have no formal charge, ie. 2
bond pairs and 2 lone pairs.
The S atom thus has 6 bond pairs, and being in Group
VI, thus has no lone pairs, because we do not wish the S
atom to have any formal charge (since the condition or
formula, that ionic charge = sum of formal charges,
is already satisfied by the two singly bonded uninegative
formal charge O atoms).
That's how you arrive at the UK presentation of the Kekule
structure of the sulfate(VI) ion :
![]()
For the US presentation, this is how you draw the Kekule
structure of the sulfate(VI) ion :
Since we do not wish to expand the central S atom's octet, thus
all O atoms must be singly bonded, and thus have 1 bond pair and 3
lone pairs, and therefore a uninegative formal charge each.
Since ionic charge is the sum of formal charges, it means we
require the central S atom to have a dipositive formal charge, to
balance out the tetranegative formal charges of the O
atoms. Accordingly, we deduce the S atom has no lone pairs,
because only when it has 4 bond pairs (which it already does, based
on the O atoms' bonding) and zero lone pairs, will it have a
dipositive formal charge, as S belongs to Group VI.
And that's how you arrive at the US presentation of the Kekule
structure of sulfate(VI) ion :
![]()
--------------------------------------
Wikipedia shows both (UK and US preferred) structures of the
sulfate(VI) ion :
![]()
as well as all the common resonance contributors of the
sulfate(VI) ion :
![]()
It is recommended that Singapore-Cambridge 'A' level students
draw the UK presentation as follows, for the sulfate(VI) ion :
![]()
-------------------------------------------
For 'A' level purposes, you will either be given the formulae
(including the charge) in the question, or are expected to know the
formulae (including the charge), and then tasked to draw the
structure.
OR (but this is not common for 'A' levels, but is even simpler
to do!)
You're given the structure, and tasked to find the formal
charges and hence overall ionic charge.
Simply indicate the formal charges, and then add them all up to
obtain the ionic charge.
For instance, if the structure given has :
a singly bonded O atom, it means it has 1 bond pair and 3
lone pairs, and thus a uninegative formal charge (since O is in
Group VI).
a doubly bonded O atom, it means it has 2 bond pairs
and 2 lone pairs, and thus no formal charge (since O is
in Group VI).
a triply bonded O atom, it means it has 3 bond pairs
and 1 lone pairs, and thus a unipositive formal charge (since O is
in Group VI).
You can extrapolate this for any atom, eg. S, N, C, etc.
-------------------------------------------
To indicate the dipositive formal charge on S, you must write it
as 2+, instead of +2. The +ve or -ve sign is behind the number.
In contrast, Oxidation States (OS) aka Oxidation Numbers (ON),
are written in brackets next to the atom, and has the +ve or -ve
sign in front of the number.
Oxidation State = Formal Charge + Electronegativity
consideration.
Thus for the US resonance contributor, OS of S = (+2) + (+4) =
+6
Thus for the UK resonance contributor, OS of S = (0) + (+6)
= +6
Hence for SO4 2-, the latin name is sulfate ion, the stock name
is sulfate(VI) ion (ie. the stock name has the OS of the heteroatom
indicated).
Note that Ionic Charge is the Sum of Formal Charges, and
also the Sum of
Oxidation States.
------------------------------------
Originally posted by H4x0ru5:
Ah thank you very much. Really enlighten me about
this.
I have a last question relating to ions.
Iodine Tetrafluoride, IF4 - is a anion.
Iodine, the central atom, has 4 bond pairs and 2 lone pairs. The
Fluorine atom has 1 bond pair, 3 lone pairs.
This gives Iodine a formal charge of -1. Each fluorine atom has
a charge of 0.
Why doesn't the compound IF4 exist, since without the extra
electron attached to Iodine, Iodine would have a 4 bond pairs, and
3 electrons attached to it. The formal charge of Iodine would
therefore be 7-3-(8/2) = 0 Why does it exists as an anion instead
of a compound ?
For it to exist as a molecule (ie. ionic charge = zero), one or
more atoms (to be precise, the I atom) would have an unpaired
electron (to be precise, 1.5 lone pairs, and the ion would be
a free radical) and is thus too unstable to exist.
IF4-
The -ve formal charge is on the central I atom (since it has a
larger atomic radius and a lower charge density and is thus more
stable, despite its lower electronegativity).
In addition, note that F (being in period 2) cannot expand its
octet, and thus to have a stable octet, it must have 1 bond pair
and 3 lone pairs, and hence no formal charge (being in Grp
VII).
Since I is in Grp VII, to have a -ve formal charge, we deduce
that it has 8 valence electrons, ie. 4 bond pairs, 2 lone
pairs.
Hence the electron geometry is octahedral, and the ionic
geometry is square planar.